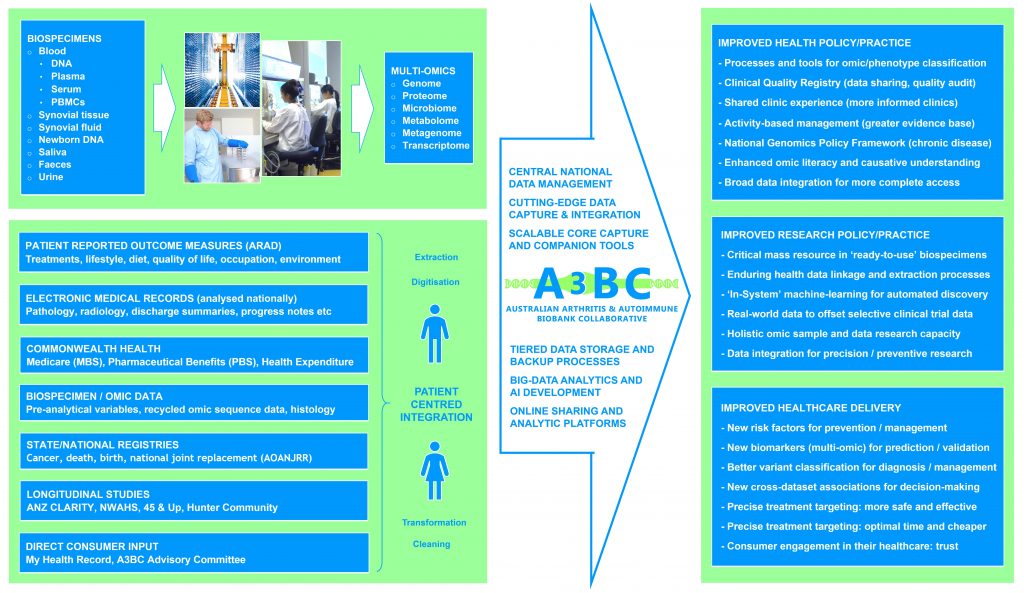
Collected Data
- Questionnaire data is collected and 0, 6, 12, 18 and 24 months, then 12-monthly for the life of the project. Data may be collected beyond these times where significant changes in disease (flares) or treatment status occur. Where participants change/switch therapies, the collection timeline will not refresh (unless specific to an approved project).
- In aligning with international best practice, and ensuring provision of high-quality longitudinal data (which randomized controlled trials cannot capture) to enable innovative research design and discovery, the A3BC has developed a minimum dataset.
- The A3BC will collect pre-analytical variables on all blood and tissue biospecimens from collection to processing to storage. The variables recorded are accordance with the Standard Preanalytical Coding for Biospecimens (version 2.0), developed by the International Society for Biological and Environmental Repositories (ISBER).
Linked Datasets
- Australian Rheumatology Association Database (ARAD) – patient-reported outcomes
- Electronic medical records (EMR) extracts for clinical/medical data
- Rheumatologist clinics for clinical outcomes and histories
- Commonwealth health data (MBS, PBS, Expenditure)
- Longitudinal/Lifecourse data (CLARITY, NWAHS, etc)
- Medical imagery and reporting (radiology, histology)
- Genomic/Omic sequence data resulting from access
- State and national registry data (cancer, death)
- Consumer data (My Health Record)
