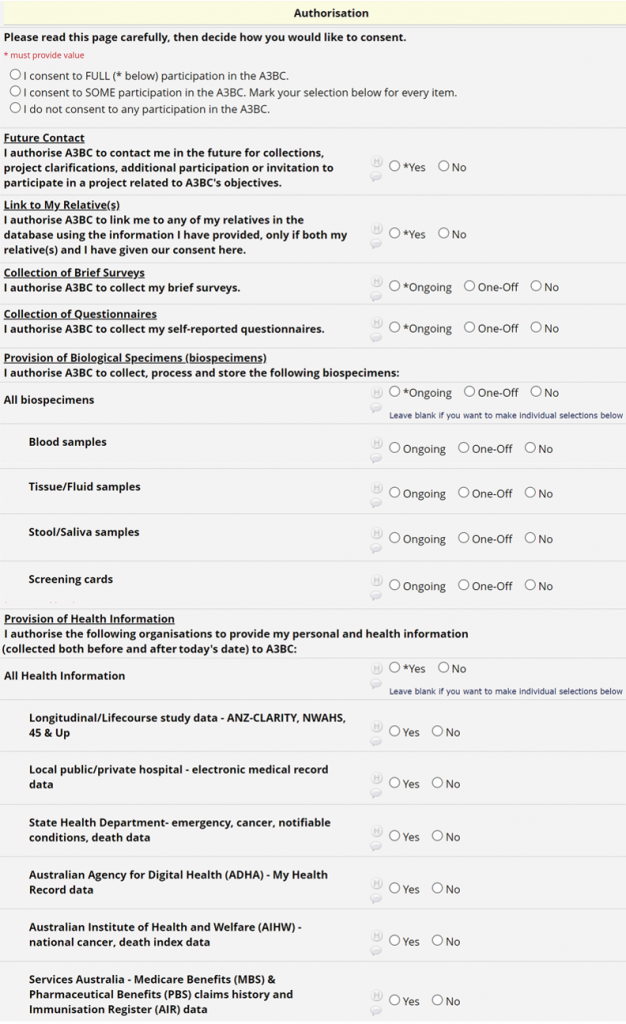
A3BC participation options have been designed to be as flexible as possible. On the Consent Form participants are given the option to participate in all, some or no/none parts of the project. For example, participants may opt-in to completing online questionnaires and providing samples, but not data-linkage, if they wish. For brief surveys, questionnaires and biological specimens participants can also choose to participate in an ongoing or one-off manner.
The A3BC uses five different consent forms for consenting participants. The relevant consent form depends on the participant’s age and disease status.
- Adult Participant Information Sheet & Consent Form (for adults with a diagnosis of arthritis or autoimmune disease)
- Overall medical research consent is required from adult participants aged 18 and over (including for Services Australia information). Participants recruited as children are required to re-consent to adult participation at 18 years.
- Adult Control Participant Information Sheet & Consent Form (for adults without a diagnosis of arthritis or autoimmune disease i.e. controls)
- Overall medical research consent is required from adult control participants aged 18 and over (including for Services Australia information).
- Child (under 14 years) Participant Information Sheet & Consent Form
- Overall medical research consent is required by the parent/guardian for children recruited under the age of 14 (including for Services Australia information)
- Child (14 – 18 years) PARENT/GUARDIAN Participant Information Sheet & Consent Form
- Overall medical research consent is required by the parent/guardian for children recruited between the ages of 14 and less than 18 (except for for Services Australia information)
- Child (14 -18 years) CHILD Participant Information Sheet and Consent Form
- Consent to release of Services Australia information must be provided by the child once they are 14 years or older
Samples
Depending on consent choice, all or some of the following will be collected at 0, 6, 12 and 24 months from the time of consent for ongoing collection (or ideally at 0 months only if one-off collection):
- A blood sample (up to 80mls or 5 tablespoons). This is generally collected during a scheduled clinic visit. Collection times will vary (under or over 24 months), to account for disease flares, treatment changes or surgeries.
- In some instances, a sample of tissue and/or fluid (incl. saliva, stool) may be collected during a scheduled procedure or within consented research.
- Newborn Screening Cards (NBSC) stored in State genetic services and archived pathology samples stored in Pathology centres are also accessible.
Data
Depending on consent choice, all or some of the following will be collected at 0, 6, 12, 18 and 24 months then 12-monthly from the time of consent for ongoing collection. The one-off survey/ questionnaire is ideally collected in the 6 months after consent.
- Brief (~10 minutes) online or paper surveys to gather basic demographic/ disease information for improved national recording of people with your condition(s). *Only collected 12-monthly.
- Online patient-reported outcome (PRO) questionnaires. Times will vary with disease flare or treatment change. The questionnaires take up to 60 minutes to complete and ask about the participant’s condition, treatments, outcomes, quality of life, diet, environment and other experiences.
- Electronic medical record (EMR) data from hospitals/clinics/pathology, State Health Department data, Commonwealth health data (including from My Health Record, the Australian Institute of Health and Welfare and Services Australia), and data from longitudinal/lifecourse studies participants are involved in (e.g. 45 & Up, ANZ-CLARITY, JAFA) will be linked.
All data will be securely stored at the University of Sydney.
Future contact
Participants will be re-contacted in three main scenarios:
- To ask participants for clarification regarding information they have already provided us under their existing consent.
- To ask participants for further information/ samples or invite them to take part in other studies. This may require a separate informed consent and would not affect participation in the A3BC.
- Participants may be contacted by a clinician to notify them of any serious incidental findings that are discovered, which have health implications for the participant or their relatives.
Linking to relatives
For research purposes, the A3BC can link participants’ data with data from their first/second degree relatives already in the A3BC database, in order to characterise environmental, genetic, microbial and immune factor relationships within families and communities. Importantly, the A3BC never shares any data between relatives and any link within the A3BC database can only occur with the consent of both parties.
