Biospecimens / Samples
Development of the sample collection-storage protocol for the A3BC Biobank network was rationalised by a number of key drivers. In particular, collection should enable a broad range of assays that are realistically envisaged in future research, and limit collection, processing or storage approaches that may hinder such assays. In other words, “future proof” the collection within current knowledge. The feasibility, storage capacity and cost of collecting and storing a large and diverse range of samples was also a key deciding factor. Due to the volume limitations of paediatric versus adult blood collection, the A3BC will deploy different blood collection strategies between these two groups.
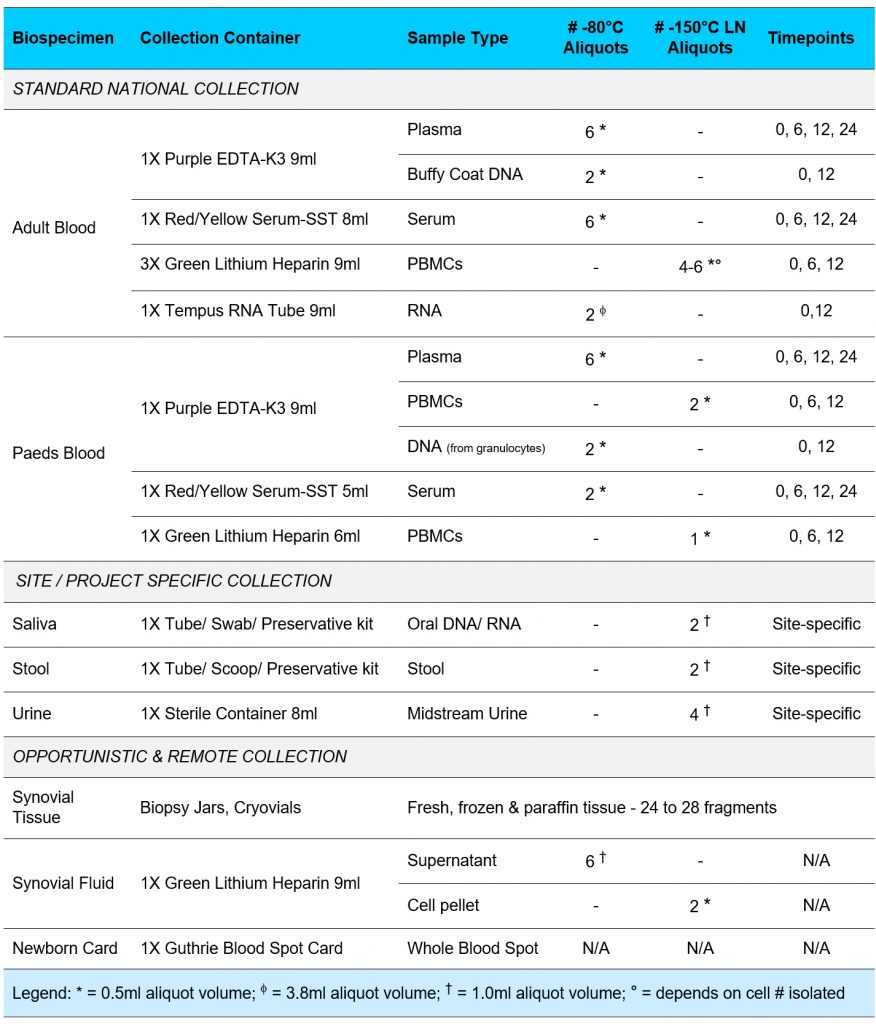
Where possible, biospecimen collection will occur concurrently within clinician care to limit further imposition on the participants. The A3BC will collect biospecimens for both incident and prevalent cases. New participants will be collected as close as possible to the commencement of treatment (baseline – 0 months), then at 6, 12 and 24 months. Ideally, baseline will be within 2 weeks of starting treatment however a 4-week window either side of significant interventions or collection of patient reported outcomes will enable the biospecimen and clinical data to be linked.
However, while regular biospecimen collection will cease at 24 months, biospecimen collection times may occasionally be adapted where significant unforeseen changes in disease (flares) or treatment status occur. Where participants change/switch therapies, the collection timeline will not refresh (unless specific to an approved project).
Other Bone and Soft Tissues
Occasionally A3BC participants may be scheduled to undergo a surgical procedure in which tissue will be removed for pathological examination and/or discarded as medical waste. Tissue samples such as these can be extremely valuable in musculoskeletal and autoimmune disease research. Tissue collection for pathology examination related to care will always take priority over research collection. For participants who are due for a routine medical/surgical procedure as part of their standard care, there are two ways that the A3BC can be notified of the participant’s wish to donate their tissue/fluid samples for research:
- The participant’s treating rheumatologist and/or orthopaedic surgeon will ask if they would like to donate their (or their child’s) tissue/fluid to the A3BC. This is completely voluntary and only done with the participant’s (or parent/guardian’s) permission. The A3BC will contact the participant’s doctors to co-ordinate the tissue /fluid collection, with no inconvenience to them. A site-specific ‘A3BC Tissue Collection and Transportation Form’ will be completed by procedural staff at the time of the procedure.
- The participant (or parent/guardian) can contact the A3BC via email or phone to discuss their (or their child’s) upcoming operation or medical procedure and determine if collection of their tissue/fluid will be feasible. The A3BC will contact the participant’s doctors to co-ordinate the tissue/fluid collection, with no inconvenience to them. A site-specific ‘A3BC Tissue Collection and Transportation Form’ will be completed by procedural staff at the time of the procedure.

Tissue types of interest include:
-
- Bone and joint tissue discarded during primary joint replacement, revision joint replacement, fusion surgery or osteotomy, such as distal femur and proximal tibia (knee replacement), femoral neck and head (hip replacement), glenohumeral joint (shoulder replacement), distal humerus and proximal radius/ulna (elbow replacement) or vertebral body (spinal osteotomy).
- Tendon or ligament tissue removed during tendon repair or debridement.
- Muscle tissue removed for muscle biopsy examination.
- Rheumatoid nodules under the skin scheduled for surgical excision.
- Excess biopsy tissue in participants with suspected vasculitis (e.g. temporal artery in suspected GCA).
